Introduction:
Diffuse Large B Cell Lymphoma (DLBL) is the most common type of Non-Hodgkin's Lymphoma (NHL) seen in Tawam hospital UAE. DLBL is a heterogenous disease arising either from a Germinal center (GC) or non- GCB cells. Molecular signature has identified 3 different groups of DLBL with significantly different prognosis (Primary mediastinal, Germinal center and Activated B Cell type). The therapy for DLBL has been without innovation since introduction of Rituximab more than 20 years ago and using R-CHOP. Attempts at improving the outcome has been uniformly unsatisfactory either by adding additional drugs, increasing the dose of drugs or deceasing the frequency of each cycle. New biomarkers and or targets are needed to improve the prognosis of patients with DLBL. Programmed Death-Ligand 1 (PDL1) has been shown to protect tumor cells by inducing T cell apoptosis (Dong et al., 2002). PDL1 targeting agents have been approved for many different types of malignancies including Hodgkin's Lymphoma. The role of PDL1 in DLBL is still under intense study. We report our findings of PDL1 expression in DLBL patients in UAE.
Methods:
Patients diagnosed with DLBL between December 1, 2019 till July 15th 2020. Patient records were reviewed in this study. Data was collected regarding patient demographics, clinical stage, International prognostic index (IPI), pathology and cell of origin {using immunohistochemistry (IHC) and Han's criteria}. PDL1 testing was performed by using FDA approved PharmaDx Dako Ab 22C3 kit for IHC on the gold standard Link 48 Dako platform. PDL1 expression was reported as Tumor proportion score (TPS). TPS in this assay was the percentage of viable tumor cells showing any perceptible partial or complete linear membranous (or membranous & cytoplasmic) staining, in the large lymphoma cells. The specimen was considered PDL1 positive if the TPS was greater than or equal to 30%(Xu-Monette et al Blood 2018). All scores were reported by single pathologist
Results
Twenty-six patients were diagnosed with DLBL in the study period. The median age was 44 years (range 18-90 years). Majority of patients (75%) were less than sixty years of age. Advanced stage disease was seen in preponderance (66%) as compared to early stage (34%) while the LDH was elevated in 65 % patients (institutional normal < 225 IU per liter). Majority of patients (60%) were poor risk with IPI 3 or higher.
Pathology review showed a uniformly elevated Ki 67 with the majority of specimens showing greater than 80 %. Molecular classification showed GCB type 50 % (n=13) as compared to non-GCB 42 % (n=11), data was not available for 2 patients. Epstein-Barr virus encoded small RNA (EBER) CISH was positive in only 3 patients (data was not available for 8 patients).
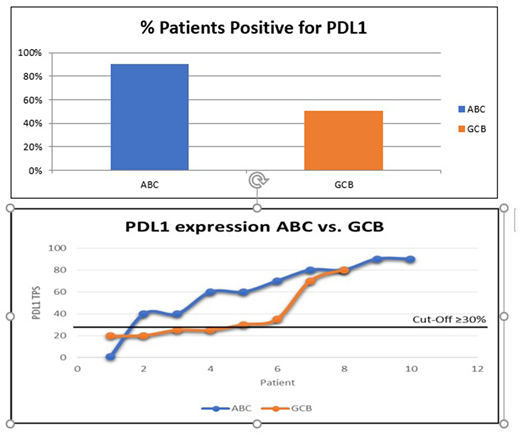
PDL1 expression was tested in eighteen (GCB n=8, ABC n=10) patients. PDL1 expression was elevated in ABC type (90%) as compared GCB type (50 %) Fig 1.
Conclusion
The median age (44 years) is at least 2 decades less than the reported western cohort. Majority of the patients had high risk disease with advanced stage and IPI. Ki 67 was uniformly elevated (>80 % in 90 % of patients). There was a slight preponderance of GCB type (50 % vs 42%). PDL1 expression in ABC type DLBL was higher as compared to GCB. This observation needs to be validated in a larger cohort.
Recent literature makes it very clear that ABC type DLBL has a poor outcome as compared to GCB type. PDL1 may offer an attractive target for treatment evaluation in ABC type DLBL.
Alam:Pfizer:Honoraria;Roche:Honoraria.McCarthy:Karyopharm:Consultancy, Honoraria;Magenta:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board;Janssen:Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board;Takeda:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board;Juno Therapeutics, a Bristol-Myers Squibb Company:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board , Research Funding is to Roswell Park, Research Funding;AbbVie:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board;Genentech:Consultancy, Honoraria;Starton:Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal